Oral bioavailability is a long-lasting issue on drug development. No one would doubt it as a breakthrough if human oral bioavailability is calculated by the molecule’s chemical structure in high accuracy. Lipinski rule-of-five1) is a rule-of-sum idea to design and choose drug-like molecules with reasonable bioavailability.
However, it is not a golden rule and some drugs out-of-criteria are on the market as bioavailable drugs. Screening based on oral availability is important for the synthesis design and lead/candidate nomination to achieve a short-term, high-probability drug discovery and development.
As it’s the age of DX, we need better tools to support the prediction of oral bioavailability.
Here is a simple and readily available free application for the calculation of PK and drug-likeness parameters: SwissADME.2) SwissADME is designed to assist computer-aided drug design (CADD).
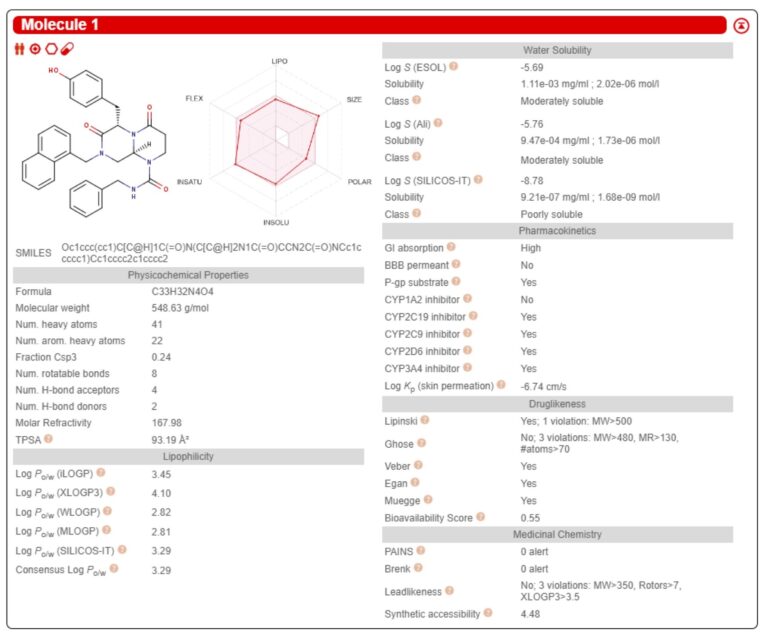
The significance of SwissADME is that it provides you a series of ADME parameters just by the submission of the chemical structures of interest by MarvinJS or SMILES. The result is summarized in a table and the data is available in csv format as well.
SwissADME can deliver the computation of a set of molecules at the same time. The list of parameters is ready to be downloaded but you can also visually inspect the important parameters online because SwissADME provides you an access to what they call Bioavailability Radar and Boiled-Egg.
Bioavailability Rader shows, in a chart, 6 parameters of drug-likeness: lipophilicity (XLOGP3), size (MW), polarity (TPSA, topological polar surface area), solubility (logS), saturation (fsp3) and flexibility (number of rotatable bonds). It allows you to filter out the molecules of reasonably orally undruggable by intuition because the 6 axes are adjusted to present the ideal one as a regular hexagon.
Boiled-Egg3) is an intuitive and visual prediction model for the evaluation of passive gastrointestinal absorption (HIA) and blood-brain-barrier (BBB) penetration. Plots of input molecules are visually analyzable on the same 2D map in the WLOGP4) and TPSA referential.
The Abbot bioavailability score5)is also available in SwissADME. The bioavailability score is the parameter which predict the probability of more than 10% oral bioavailability in rat based on PSA (potential surface area) and Lipinski rule-of-five. This parameter falls on four classes of probabilities (11%, 17%, 56% or 85%) and allows to filter off the molecules of cell-permeability issues.
The combination of these tools would help us to rule out the unfavorable molecules from their chemical structure. However, the accuracy of the prediction needs to be increased to select the candidate for clinical trials as well as not to exclude the real candidate by misprediction.
We are internally working on the prediction of ADME parameters from the structural information of PepMetics®. Machine learning is giving us a fabulous chance to construct the highly reliable model for the selection of the lead and the candidate. If you are interested, please contact us to have a fruitful discussion.
1) https://doi.org/10.1038/srep42717
2) https://doi.org/10.1016/S0169-409X(96)00423-1
3) https://doi.org/10.1002/cmdc.201600182
4) https://doi.org/10.1021/ci990307l
5) https://doi.org/10.1021/jm0492002