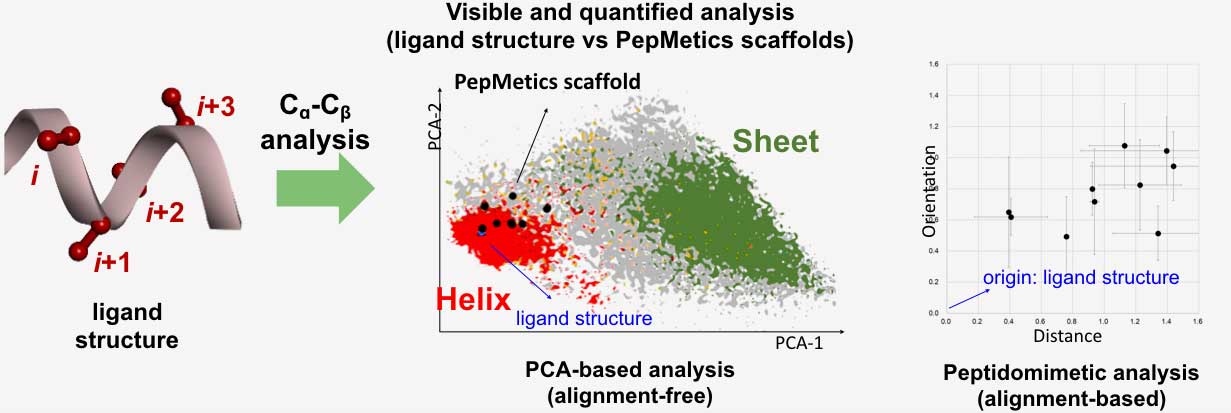
PepMetics™ Technology enables the Scaffold Mimetic Approach for Structure-Based Drug Design (SBDD).
While conventional SBDD uses pharmacophores to fit into the binding site of the partner pocket, PepMetics can mimic the scaffold structure of the ligand peptide and attach corresponding amino acid side chains of the ligand.
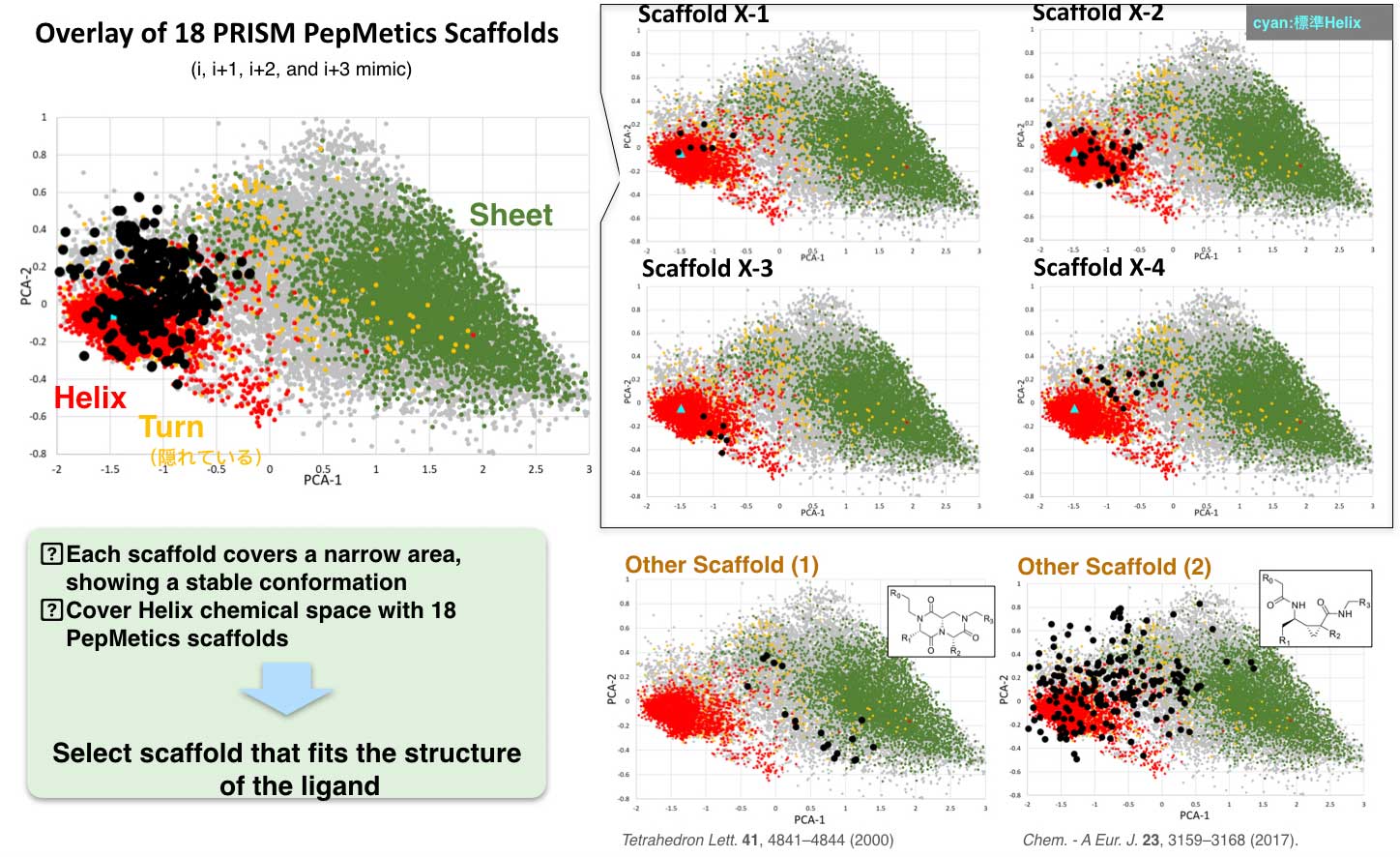
The more than forty scaffolds included in the PepMetics Library covers a wide range of helical structures such as a standard, bent, or partially unfolded helix (i.e. represented by the red area in the graphic below). Each scaffold covers a specific area of the helix, ultimately replicating the necessary structure of a natural helix.
The figure shows a chemical space of peptide structures extracted from proteins based on side-chain Cα-Cβ bond analysis. The red and green dots represent conformation distributions of α-helix and β-sheet structures in proteins, respectively. The black dots, the possible conformations a PepMetics scaffold can take, are positioned on the helix region, which means that the scaffolds are excellent mimetics of α-helix structures.
If the black dots are broadly distributed, the compound’s binding ability is compromised by entropic loss. When a close relationship is observed in the plot, the scaffolds are likely to keep their shape and bind well with superior selectivity to the matching target.
Each of PRISM’s scaffolds has specific conformations and covers a narrow area. A cluster of 18 PepMetics scaffolds cover the entire helix chemical space. PRISM selects scaffolds which are suitable for the target ligand structure.